Dear TechNet community, this year marks the 50th anniversary of the creation of the Expanded Programme on Immunization (EPI). WHO and other partners will be commemorating this with various events and publications throughout the year.
One idea being considered is a short video on the evolution of the immunization supply chain (ISC), looking at the key innovations, technologies, events, and milestones that have enabled the ISC to support the demands of growing populations and expanding national vaccine schedules. This has got me thinking about what the most significant milestones and innovations might be, and specifically how they could be represented in a timeline of key dates.
Below is a no-doubt incomplete (and also slightly biased;-) list that I have come up with. I would be very interested in hearing the thoughts of other TechNet members on this. Milestones in the early years are particularly light… Your suggestions would be very welcome!
- 1974 WHO launches the Expanded Programme on Immunization (EPI)
- 1990 First TechNet Consultation
- 1996 Vaccine vial monitor (VVM) first used
- 2000 Launch of the Global Alliance for Vaccines and Immunisation (Gavi) and the Bill & Melinda Gates Foundation
- 2002 (?) WHO PQS prequalification of AD-syringes
- 2008-2013 Project Optimize
- 2008 (?) Prequalification of first solar direct-drive (SDD) vaccine refrigerator
- 2009 WHO and UNICEF launch the Effective Vaccine Management (EVM) assessment tool
- 2011-2020 The Global Vaccine Action plan (GVAP) and the Decade of Vaccines
- 2014 First vaccine (MenAfriVac) to be used in CTC during immunization campaign
- 2018+ Launch of the Gavi Cold Chain Equipment Optimization Platform (CCEOP)
- 2020 (?) WHO PQS prequalification of first freeze-preventive vaccine carrier
- 2020 Launch of the Immunization Agenda 2030
What is missing:
- The transition from paper-based to digital immunization registries and stock management systems; this is undoubtedly highly significant but how to include this in the above?
- The development and deployment of new temperature monitoring devices, such as electronic temperature loggers, freeze indicators, and shipping indicators; user-programmable data-loggers; and remote temperature monitoring devices (RTMDs) and systems. Beyond the "first" product to be prequalified in these PQS categories, I wonder if there is a "first" national deployment that could be cited as the milestone in the above timeline?
- The move towards net-zero supply chains
- The transition from kerosene-powered to soloar refrigeration
- Solar-powered cold rooms and freezer rooms
Beyond the innovations and milestones above, I am also wondering what could be suggested as good prospects for future innovations that will continue to transform the ISC landscape, for example:
- Unmanned aerial vehicles (UAVs)
- Traceability (barcodes, blockchain, etc.)
Looking forward to some stimulating and illunimating responses.
Dear Dan
- The first TechNet consultation was held in Cyprus in 1989
- The AD syringes were not initially PQS prequalified because the PQS process was invented later by Umit (around 2005 I think) – the AD syringes were actually independently tested by WHO approved laboratories and found to meet WHO Performance specifications and then listed in the WHO/EPI “Product Information Sheets” or PIS a catalogue of equipment managed by WHO which UNicef SD relied upon for its procurement / THe PQS process eventually replaced that
- The PIS was an important tool for many years for logistics officers and programmles around the World to identify and select cold chain equipment and injection materials
- Peter Evans is the person who drove the introduction of AD syringes - the real game changer howerver happened first WHO/UNICEF and other agencies adopted the first call for the “Safety of Injection “ and introduced the concept of "bundling" (whereby no dose of vaccine should be supplied without its AD syringe)- Gavi then adopted this policy of bundling and made it possible to be implemented
- The VVM history is well documlented in the attached document. PATH was the leader in VVM development togethr with the company Lifelines - The game changer however was when Gavi decided to only finance the procurement of vaccines with VVMs - THis pushed manufacturers to make the effort to affix VVMs on their vaccine vials (aroudn 2001) and also drove unicef SD to request VVMs in their tenders
Note : the BMGF was not created in 2000- It existed before and had already funded a major children immunization programme through PATH (100 million dollars over 10 years) which Mark Kane a former CDC and WHO staff led for many years. This programme was launched in the mid-late 1990's I believe and was instrumental in facilitating the discussions around the creation of and Launch of Gavi (the meeting of the protoboard of Gavi was held in July 1999 in Seattle at the invitation of Path and the Children Vaccine Programm which led Mr Bill Gates Senior to approve a US $ 750 million grant to create GAVI
best
Michel
Hi Dan!
Please, find attached a slide of mine with some steps. I am open to discusss them.
Thanks!
Modibo
Here are a few suggestions:
1976 John lloyd outlies the strategy for how vaccine delivery and storage could be more systematically managed
1982? introduction of long life cold boxes
1999 Development of strategies for safe injections
2003 direct drive solar refrigerators
Daniel,
I think that's a good list you've assembled to which I would add the following: The “Guidelines on the international packaging and shipping of vaccines,” first developed by WHO in 1981, will seem like ancient history and pale in comparison with images of tangible, photogenic objects. However, just as the successful Smallpox Eradication Program wisely paid close attention at its inception to the varying quality of smallpox vaccines in use around the world, these WHO guidelines played (and continue to play) an essential role in establishing vaccine shipping and packaging requirements which guide manufacturers and suppliers for that most crucial first link in the cold chain, through which all vaccines supplied by UN agencies must travel.
Thaks it is well summrized ,and Nice tip to us for commemorating the key inovation ,
Can you please indicte COVID-19 vaccine supply chain managment and it develeopment process as the new emegrign innovation ?
Thanks Dan for this initiative below is my contribution
I believe the evolution of EVM requires its own place here i.e, from the era of VMA in the early 2006/7 ( in AFRO) to EVSM (global certifications) and then EVM to EVM 2.0 a logtof effort and knowledge from multi-disciplinary got involved on EVM and EVM 2.0
I also believe the transition from PIS to PQS with its differences deserves it own millstone.
The transition from “paper based temperature monitoring systems” such as fridge tag , CCM to the electronic system such as 30 DTRs, freeze Tags , Q-Tags etc also need its own place
on more details we can also talk about evolution of equipment , from absorption to Solar battery drive to solar direct drive .
On passive equipment front , we can also cite long range passive devices , introduction of freeze free cold boxes and vaccine carries.
Dereje
The BIG fat juicy disclaimer: I’m biased. As the manufacturer of the Fridge-tag my points below are subjective. Yet I still think they are worthwhile mentioning.
Some 18 to 20 years ago, the Fridge-tag was introduced as the first data logger for fridge monitoring (storage of vaccines). Prior to that there has been emphasis on the monitoring of shipments (rightfully so). Yet without a solution or even standard for storage monitoring. The conception of the Fridge-tag was based on an open conversation between Umit Kartoglu of WHO PQS, key cold chain practitioners and Andrea Berlinger. In this brainstorm it was verbally discussed that a monitoring solution for fridges was desirable, and what the key functionalities of such fridge monitor should be.
There was no (yet) a PQS Standard. There was no RFI, RFP or RFQ. It simply was a conceptual idea that made sense and the benefits were evident. A few months after that initial discussion Berlinger introduced the first Fridge-tag to WHO and UNICEF.
Currently the yellow Fridge-tags are used in the vast majority of fridges and freezers in LMIC. Fridge-tags are making a significant contribution to the immunization supply chain. More than a million (!) Fridge-tags have collectively been safeguarding vaccines in the field in the past two decades.
New Horizons said in the Industry Consultation in 2022 (Seattle): ‘…if you check a fridge in any LMIC, the Berlinger Fridge-tag is almost always guaranteed to work’
To wrap it up: an overview of 50 years immunization supply chains is not complete without the development, prequalification, and widespread adoption of data loggers such as the Fridge-tag. Because of its wide use and impact, and also as an example of how innovation can work - based on a concept (rather than a RFI/RFP), I would say that the Fridge-tag has been a game changer in the immunization supply chain. And therefore, the Fridge-tag deserves to be included in the overview.
Dear Daniel,
this is a very good iniciative. I went through others valuable comments and what I think remaining for me to comment is the EVSM (Effective Vaccine Store Management) iniciative focused at National levels and in that time providing also certificates fro the stores that were reaching criterias and countries made great effort to improve their supply chain at national level. I think it was prepared and tested in 2003 and then used broadly from 2005. Umit Kartoglu can help on the right year.
After that it was upgraded to the EVM 1 and then EVM 2 tool.
What about the "shake test"? Based on that a lot of vaccines have been saved, or discarded and saving children from being vaccinated from non effective vaccines.
That's for now. I will comment again if I remember something else.
Hope in the end we will have a good story.
Hi Dan and colleagues,
I'm super excited to see this TechNet thread and have a few of the key milestones to add to your list. I'm also providing some useful citations that can substantiate some of the dates I provide. Under each article, I state the dates I think most important; however, some of the articles have more detailed timelines in case any readers want to explore further.
Robertson J, Franzel L, Maire D. Innovations in cold chain equipment for immunization supply chains. Vaccine. 2017;35(17):2252‒2259. https://doi.org/10.1016/j.vaccine.2016.11.094
This article contains key dates for solar direct-drive refrigerators, long-term passive cold boxes, and equipment with user-independent freeze prevention.
Solar direct-drive refrigerators
- 2004: Market emergence of solar direct-drive refrigerators.
- 2010: First solar direct-drive refrigerator prequalified by the World Health Organization (WHO) Performance, Quality and Safety (PQS) program.
Long-term passive devices
- 2011: Market emergence timeline of long-term passive cold boxes.
- 2012: First long-term passive cold box prequalified by WHO PQS.
Equipment with user-independent freeze prevention
- 2016: 23 mains and solar direct-drive refrigerators certified with Grade A freeze prevention.
Lloyd J, Cheyne J. The origins of the vaccine cold chain and a glimpse of the future. Vaccine. 2017;35(17):2115‒2120. https://doi.org/10.1016/j.vaccine.2016.11.097.
This article contains key dates regarding the origin of the vaccine cold chain.
- 1974: WHO established the Expanded Programme on Immunization.
- 1974: Vaccine cold boxes and carriers were developed to transport vaccine
- 1977: Innovations such as participative training (replacing traditional presentations) were used to develop training courses for senior program managers and regional- and district-level staff.
PATH. A HealthTech Historical Profile: Vaccine Vial Monitors. PATH; 2005. https://media.path.org/documents/TS_hthp_vvms.pdf
This article contains key dates for vaccine vial monitors.
- 1994: The TechNet consultation to WHO recommended that vaccine vial monitors be included on all vaccines, beginning with oral polio vaccine.
Kumar S. Breakthrough vaccine carrier solves an invisible challenge to vaccine potency. PATH; 2022. https://www.path.org/our-impact/articles/breakthrough-vaccine-carrier-solves-an-invisible-challenge-to-vaccine-potency/#:~:text=Redesigning%20the%20vaccine%20carrier,%E2%80%93based%20phase%2Dchange%20material
- 2016: PATH put a freeze-safe reference design into the public domain.
- 2017: A vaccine carrier manufactured by AOV International using the freeze-safe reference design became the first freeze-preventive carrier to obtain WHO PQS prequalification.
WHO. Freeze-Free Vaccine Cold Box: E004/057. https://apps.who.int/immunization_standards/vaccine_quality/pqs_catalogue/LinkPDF.aspx?UniqueID=a7dafe31-418b-48ad-9385-acda9036426a&TipoDoc=DataSheet&ID=0
- 2020: Qingdao Leff International Trading Company became the first manufacturer to receive WHO PQS prequalification for a freeze-preventive cold box.
PATH. Technologies for injection safety: Historical overview and current status. Fact sheet. PATH; 2021. https://media.path.org/documents/Injection-safety-tech-fs-Apr2021_4-29-21.pdf?_gl=1*sdgh5s*_gcl_au*MTM0NTE2NjQ2LjE3MDIzMzE0MDA.*_ga*MTU2NDI1Njg2Ni4xNjk0NDY5Mjky*_ga_YBSE7ZKDQM*MTcwNTk3MDgxMS45OC4xLjE3MDU5NzIxMzkuNjAuMC4w
- 1992: PATH advanced a design for an autodisable syringe that became the first commercialized autodisable syringe product when launched by BD as the SoloShot™ in 1992.
Mvundura M, Frivold C, Janik Osborne A, et al. Vaccine Innovation Prioritisation Strategy: findings from three country-stakeholder consultations on vaccine product innovations. Vaccine. 2021;39(49):7195‒7207. https://doi.org/10.1016/j.vaccine.2021.08.024
- 2017: The Vaccine Innovation Prioritisation Strategy (VIPS) was launched by Gavi, the Vaccine Alliance, WHO, the Bill & Melinda Gates Foundation, the United Nations Children’s Fund (UNICEF), and PATH—known collectively as the VIPS Alliance.
In 2017, the first WHO energy harvest control was prequalified.
Giersing B, Shah N, Kristensen D, et al. Strategies for vaccine-product innovation: creating an enabling environment for product development to uptake in low- and middle-income countries. Vaccine. 2021;39(49):7208‒7219. https://doi.org/10.1016/j.vaccine.2021.07.091
This article contains key dates in the development of autodisable syringes, jet injectors, the Uniject™ injection system, and vaccine vial monitors.
Autodisable syringes
- 1999: WHO/UNICEF/United Nations Population Fund issued a joint statement on injection safety, requiring exclusive use of autodisable syringes, both in routine and mass immunization campaigns.
- 2019: A second joint statement stated that UNICEF would not procure non-autodisable syringes for any immunization activities.
Jet injectors
- 2013: PharmaJet Stratis was prequalified.
Uniject injection system
- 2003: BioFarma received WHO prequalification for their tetanus toxoid vaccine in the Uniject injection system.
Vaccine vial monitors
- 1999: A WHO/UNICEF joint statement was released advocating for the use of vaccine vial monitors on all vaccines.
- 2002: Gavi stipulated the inclusion of vaccine vial monitors on all vaccines purchased through the Vaccine Fund.
PATH. A HealthTech Historical Profile: The Uniject Device. PATH; 2005. https://media.path.org/documents/TS_hthp_uniject.pdf
This article contains key dates in the development of the Uniject injection system.
- 1987: PATH invented a new design featuring a collapsible blister and the Uniject injection system was born.
And of course, Dan, you need to include the creation of the TechNet-21 website and conference. I recall you presented the dates in Panama.
Thanks,
Joe
Dear colleagues,
I would like to add my bid to Dan’s question on the most important innovations and milestones in the evolution of the immunization supply chain since 1974 with some referrals to other colleagues who already posted on this.
Although PQS is mentioned through the prequalification of AD syringes, solar direct-drive (SDD) vaccine refrigerators and freeze-preventive vaccine carriers, (as rightly brought up by Dereje) I strongly believe PQS should be considered an important milestone on its own. With the help of PQS programme, performance, quality, and safety aspects of immunization devices and tools were brought into effect with a comprehensive system through specifications, verification protocols, and most importantly standard operating procedures defining the modus operandi of the whole system. The PQS programme was announced at the Antalya TechNet Conference during 23-25 March 2004 and was said that it is expected to be launched at the end of 2006. The very first set of product specifications and verification protocols of E006 (temperature monitoring devices) from PQS was released on 9 January 2007 and the first prequalified products naturally were temperature monitoring devices. With its launch, PIS was discontinued.
I agree with the launch of Gavi being considered as a milestone, but despite its critical role in the realization of many immunization programme-related advances, listing the foundation of BMGF as an innovation or a milestone would be a mistake because of its private nature.
I also agree on the importance of EVM but we should not forget that EVM is built upon EVSM and VM programmes and its principles and tools that were brought to life by WHO and UNICEF in 2004. The EVM tool is based on the EVSM and VM assessment tools. Unfortunately, EVM initiative did not mention its predecessors EVSM and VM at all. As Erida Nelaj pointed out EVSM/VM evolution to EVM, WHO/UNICEF EVSM and VM initiatives should be considered as an important milestone in the field of immunization.
Again, as mentioned by Erida Nelaj, the shake test should be listed. Although the shake test was in use for years before its validation study (published in February 2010 doi:10.2471/BLT.08.056879), there were mistakes in the initial WHO protocol (like suggestion testing freeze-suspected vials against never-frozen vials) and no scientific validation has been done. In this sense, the shake test validation study is a landmark study and deserves to be listed here.
Lastly, I would like to refer to Hendrik’s contribution on the inclusion of the Fridge-tag. In a PDA supply chain meeting held in Collonge, Germany, during a lunch with temperature device manufacturers I spoke about the problems of temperature monitoring in vaccine refrigerators (thermometers were used for this purpose, which thermometer is not a monitoring device) and explained the functions of a dream device I had in mind and asked whether the development of such a device is possible. They all said yes yes, but nothing so concrete (well, with all competitors on the table, I did not expect more). The same year in December, Andrea Berlinger visited me at WHO and said she had a gift for my Xmas tree and put the prototype Fridge-tag on my desk. I was watching the exact device I explained months ago at the Collonge Conference. Since we did not have any PQS specifications and verification protocol, it took some time to develop and the very first device to be prequalified was Berlinger’s Fridge-tag in the coming year. I strongly believe, the 30-day electronic refrigerator logger to be generic was a revolutionary device in the immunization supply chain and strongly believe it deserves to be included in the list.
Warmest regards,
UMIT
Dear experts
I think the main milestone for Colombia was the PIS 2000, with this support in 2004 the Ministry of Health began to change all the equipments in all the country to ILR instead of domestic refrigerators. Before that document, the vaccine was exposed to daily excursions. In the document mentioned by Joseph Little https://doi.org/10.1016/j.vaccine.2016.11.097. appear the Universidad del Valle in Colombia where were made some proofs and was demostraded that although the vaccinator put bottles of water inside the appliance, the temperature arise up to 22°C punctual and restablesh its condiction 30 minutos later.
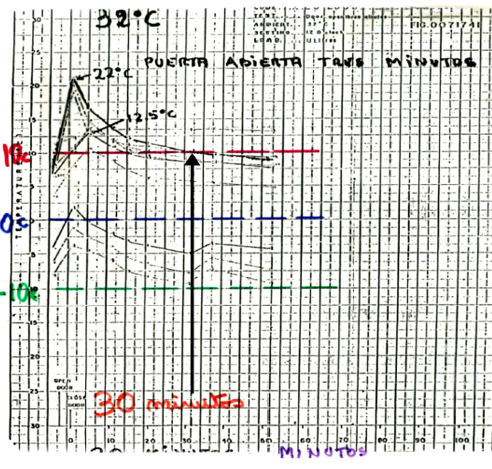
The second milestone was the SSD. Colombia has a lot of humid jungle and areas not interconnected with electrical grid. To reach those population the Ministry of Health bought solar refrigerators tested in the desert but the high humidity of our zones destroyed them, then was a failed. Furthermore the bad selection on the batteries with its regulator of charge accelerated the damage of the batteries and again no equipment to storage vaccines.
Our third milestone was the beginning use of Rotavirus and Nemo in the EPI because those vaccines spiked the requirement of volume in 400%. The Ministry of Health took the decision of build cold rooms in the main citys of the country and build a warehouse refrigerated with 600 pallets of capacity (1.20m. w x 1.20m. l x 2.0m. h). Those decision optimised the supply chain with the Covid-19 to garantee the cold chain of the 22 vaccines of the our EPI plus the Covid-19 in all the country.
Best regards
Many thanks to all for your insightful responses. I now have a much clearer picture of the ISC evolution :-)
I found hearing about Colombia's national perspective from Rafael very interesting. He gave three milestones:
- 2000 PIS & from 2004 Colombia MOH "began to change all the equipments in all the country to ILR instead of domestic refrigerators"
- Introduction of solar direct-drive (SDD) vaccine refrigerators - replacing failed solar battery projects (if I understand correctly)
- New vaccine introduction (rotavirus and pneumococcal) - increased requirement for cold chain capacity and necessitated construction of new cold rooms that were later used in the COVID-19 response
I would be very interested in learning about other country perspectives on what their key milestones might be...? Would they be aligned with the global milestones discussed above or completely different?
- Page :
- 1
