Dear TechNet community,
Poor power conditions are a major challenge to maintaining the vaccine cold chain in many LMICs, necessitating the development of specialized CCE such as ILRs and SDD refrigerators to help keep vaccines at safe temperatures in areas with limited or no mains power. Even in places with an electrical grid connection or a generator, intermittent power can lead to ILRs running out of holdover, and erratic voltages can damage many types of medical devices and equipment.
Despite these well-known issues, to date, data on power conditions in LMIC health facilities have been largely anecdotal or small-scale, which impedes evidence-based policymaking. To quantify power availability and quality challenges, Global Good – in close partnership with Nigeria’s National Primary Health Care Development Agency (NPHCDA) and Kenya’s National Vaccines and Immunization Program (NVIP) – has compiled an analysis of mains power data reported over nearly 18 months by WHO-prequalified ILRs operating in health facilities across Nigeria and Kenya. We are sharing this analysis in hopes of informing tangible improvements in both the performance and reliability of mains-powered CCE, as well as other priority medical devices and equipment utilized in health facilities within LMICs.
This analysis, co-authored by NPHCDA and NVIP officials, is based on approximately 96,000 facility-days of mains power data collected over a nearly 18-month period from ILRs operating in more than 300 health facilities spanning both countries. These power data and other information collected by the ILRs belong to the respective countries; Global Good has been granted access to the data to enable collaboration with pertinent national and global stakeholders on tools to better utilize CCE data and make it actionable. This overview of grid quality realities on the ground shows that multi-day interruptions are common, and that proper protection for medical equipment is essential given the widely varying line voltages experienced at most health facilities.
The initial analysis in this paper focuses on general power availability and quality at health facilities, with a few specific implications for CCE at those facilities including:
- 68% of the monitored devices in Kenya and 92% of those in Nigeria experienced power outages in excess of 48 hours. Therefore, long holdover times provide additional safety, even at those facilities with generally reliable mains power.
- Voltages fluctuate significantly, and stabilization can increase the ‘usable power’ availability at many health facilities – only 32% of the devices in Nigeria had access to in-range power (i.e., within 10% of nominal voltage) more than 30% of the time. However, with PQS-defined extended range voltage stabilization (i.e., 110 to 278 V), 58% of devices would have access to ‘usable power’ more than 30% of the time.
- Damaging high voltage events are common and can persist for hours or days – the graphs below show example voltage profiles from Kenya and Nigeria that would require protection between the socket and CCE (or other medical devices and equipment) to prevent damage to the electronic controls over time. These traces show data measured every 10 seconds, illustrating sustained voltages both above 400 V and below 100 V, and rapid fluctuations in and out of the CCE’s usable voltage range.
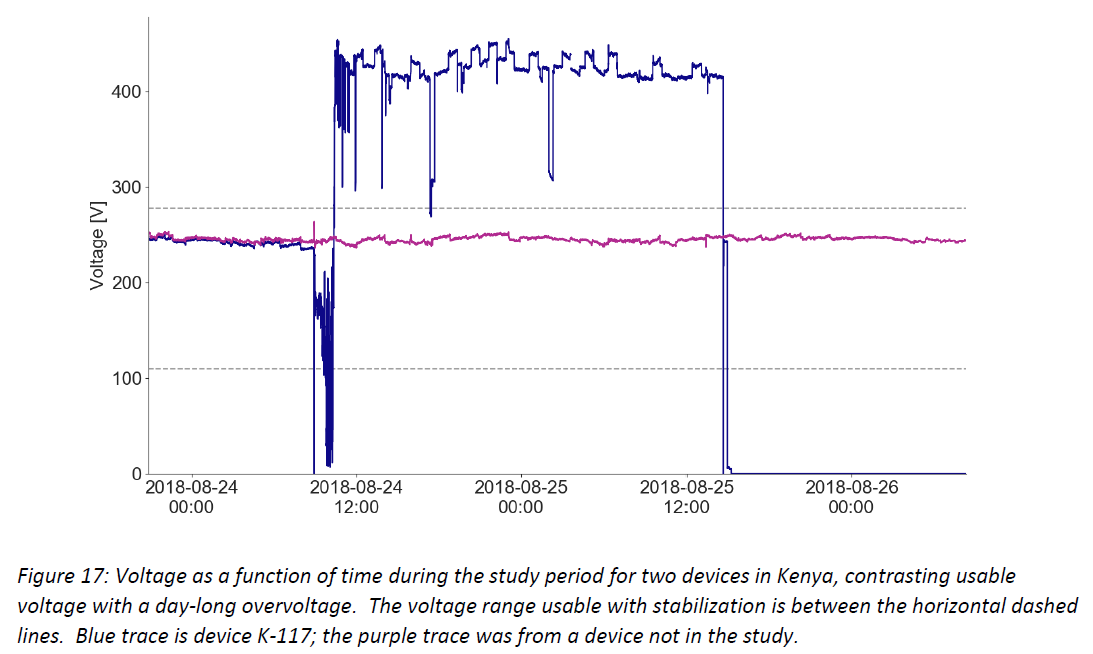
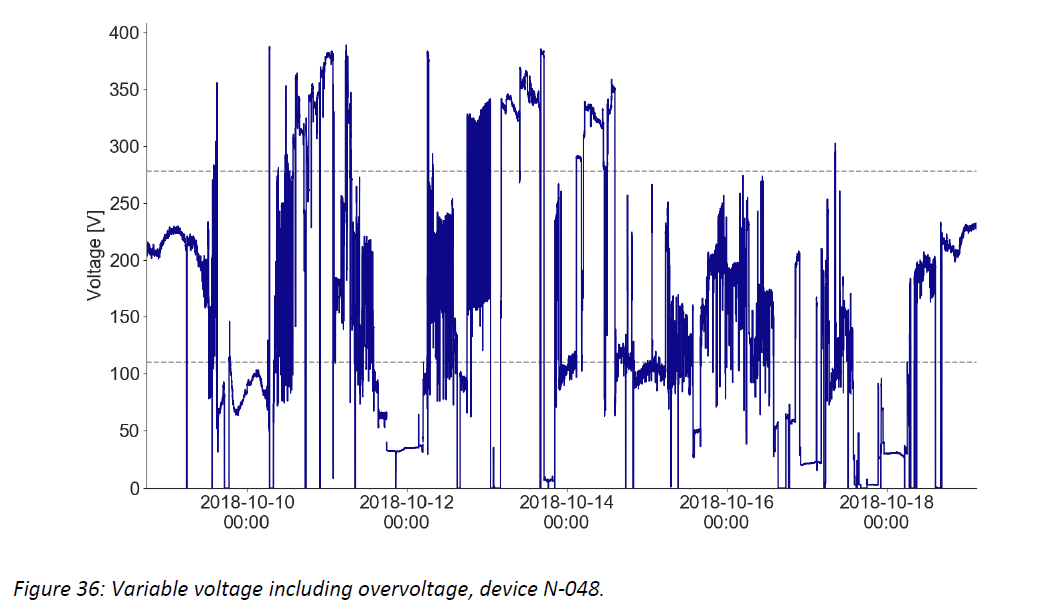
Additional graphics and discussion are in the full document, and Global Good will continue analyzing data and sharing conclusions as more information is collected.
We hope this analysis will prove valuable for specifications- and standards-setting bodies, equipment designers and equipment purchasers, and we look forward to a vibrant discussion with the TechNet-21 community via this forum.
We wish to acknowledge and extend our sincere appreciation to NPHCDA and NVIP for supporting and co-authoring this analysis. We also want to acknowledge Qingdao Aucma Global Medical Co. for producing the WHO-prequalified ILRs that collected the study data, and eHealth Africa, Caroga Pharma Kenya Ltd, and Fenlab Ltd. for installation and service support in the two countries.
The document is also availble here: http://power.2to8.cc/Power_Quality_Challenges_In_Low_Resource_Settings_2019.pdf
Best regards,
Jenny Hu
Senior Engineering Lead
Global Good
- Page :
- 1
