This page describes how the GYFLaN is structured, activities it undertakes, and how it is coordinated. At the bottom of the page are links to Members-only pages for the GYFLaN and the LTWG.
About the GYFLaN
The GYFLaN is an international coalition of laboratories routinely testing for yellow fever (YF) as part of active national YF surveillance programmes in endemic areas of the African and Eastern Mediterranean regions and the region of the Americas. These laboratories individually serve front-line YF diagnostic testing functions for YF surveillance in their respective countries, critical in identifying YF outbreaks. Working closely together, the GYFLaN laboratories, the country disease surveillance and immunization programmes and their partners, and the Ministries of Health coordinate testing, including confirmatory YF diagnostic testing at the regional level, communication of results, case classification, and strategic/preventive programmatic responses. The efficiency of this process and accuracy of results are paramount as a single confirmed YF case can be enough to initiate an outbreak response. The hierarchy of laboratories is represented below, associated with this list of GYFLaN laboratories.
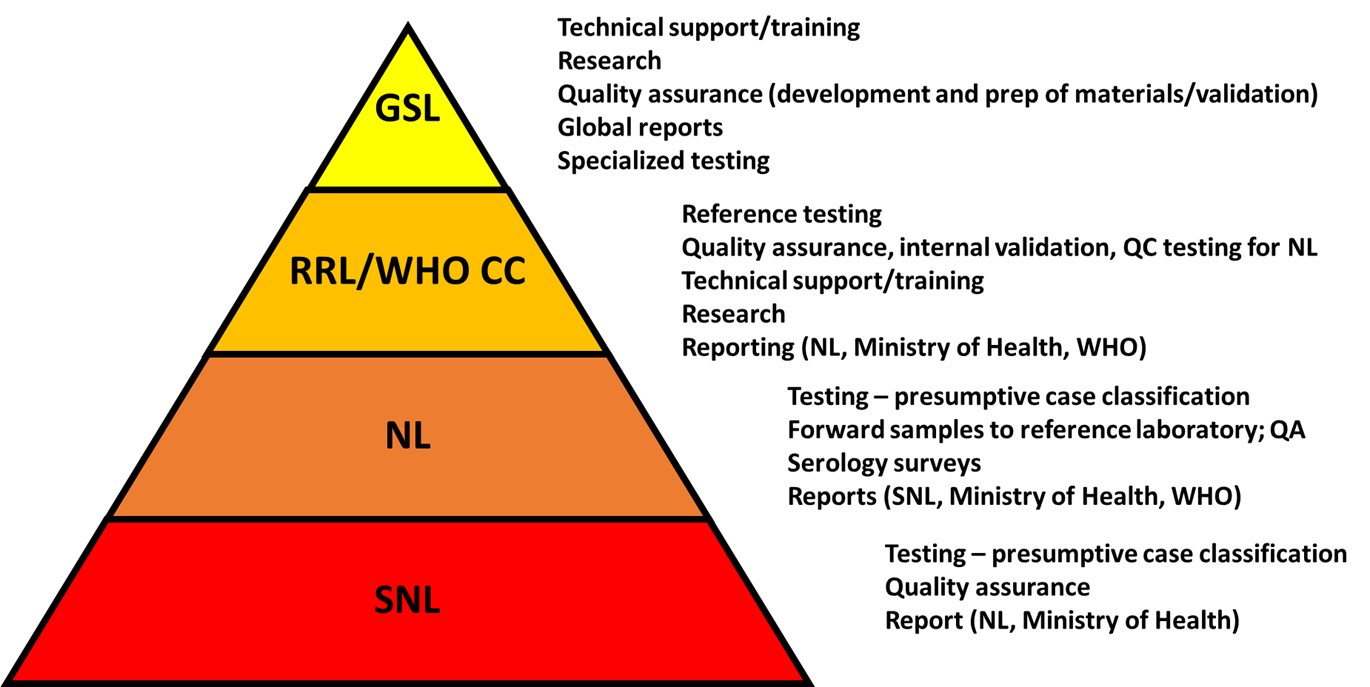
Figure. Structure and function of the GYFLaN. Abbreviations: GSL : Global Specialized Laboratory; RRL : Regional Reference Laboratory; WHO CC : World Health Organization’s Collaborative Centers; NL : National Laboratory; SN :, Sub-National Laboratory
Activities of the GYFLaN
There are three broad areas of activities that GYFLaN laboratories carry out in support of the YF surveillance programmes. These are listed and discussed in detail in the WHO YF Laboratory Manual. Briefly, these are:
- Timely and accurate laboratory testing for YF surveillance and early detection of YF cases to allow rapid implementation of control measures. Primary detection methods are real-time reverse transcription polymerase chain reaction (RT-qPCR) for detection of viral RNA and detection of IgM antibodies to YF. Confirmatory detection of antibodies uses plaque-reduction neutralization test (PRNT).
- Communication and documentation of laboratory results and data at local and national levels. Weekly or monthly reports are provided to WHO.
- Participation in WHO quality assessment and accreditation programmes. Proficiency testing via external quality assessments and annual WHO accreditation are required for GYFLaN laboratories. Quality control is also maintained by comparison of NL test results with those of their designated RRL for a percentage of the samples. More details can be found on the Lab performance monitoring page.
Coordination
The GYFLaN is coordinated by the World Health Organization (WHO), where the African Region (AFR), the Eastern Mediterranean Region (EMR), and the Region of the Americas (AMR) Regional Coordinators (RC) work closely with the Network laboratories and with the GYFLaN Coordinator at WHO Headquarters (WHO HQ). Guidance and assistance are provided by the Eliminate Yellow Fever Epidemics strategy Laboratory Technical Working Group (EYE-LTWG), the activities of which are partially funded by Gavi, the Vaccine Alliance (Gavi). More information on the EYE-LTWG can be found on the EYE strategy & LTWG page. Procurement and distribution of essential standardized laboratory equipment and reagents are coordinated through WHO along with other partners such as Gavi and UNICEF Supply Division.
